Regulatory
Support
DMF for Clinical Trials
Synecta™ T1 Activation in Clinical Use
The University of Pennsylvania has amended an ongoing clinical trial to initiate a new cohort using Synecta as an alternative to bead-based activation in CAR-T cell manufacturing. As part of the review process, the U.S. FDA evaluated the Drug Master File (DMF) for Synecta—our lead cell-derived nanoparticle (CDNP) product for T cell activation and expansion—and authorized the University to proceed.
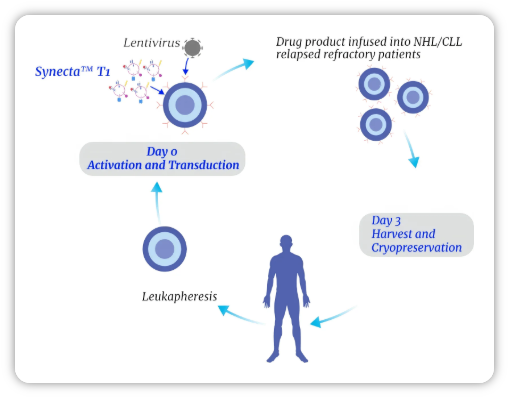
Synecta is employed in a 3-day expansion process, paired with Day 0™ transduction. Engineering runs replacing synthetic beads with Synecta demonstrated unprecedented yields of CD19/IL-18-positive CAR-T cells after only three days of culture.
